Abstract
Background:
JAK2 mutation-enriched myeloproliferative neoplasms (MPN) might progress into blast phase disease (MPN-BP), operationally defined by the presence of ≥20% blasts in the peripheral blood (PB) or bone marrow (BM) (Blood 2016;127:2391). In the current study, we reviewed consecutive cases of MPN-BP from the institutional databases of the Mayo Clinic, Rochester, MN, USA and the AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM), with the intent to examine i) survival trends, ii) treatment response rates and outcome and iii) risk factors for survival, including the impact of chemotherapy and allogeneic stem cell transplant (ASCT).
Methods:
Diagnoses of MPN, including primary myelofibrosis (PMF), polycythemia vera (PV), essential thrombocythemia (ET) and MPN-BP were according to WHO criteria (Blood 2016;127:2391). Treatment response to MPN-BP was assessed according to conventional criteria (JCO 2003;21:4642). Statistical analyses considered clinical and laboratory data collected at the time of documented disease transformation into MPN-BP. Survival was calculated from the date of leukemic transformation to the date of death or last contact. In order to examine survival trends, comparative analysis was performed based on year of leukemic transformation.
Results:
Mayo Cohort (n=248):
Patient characteristics at time of leukemic transformation: The Mayo Clinic cohort consisted of 248 consecutive patients (median age 67 years; 65% males) with MPN-BP, including 118 (48%) post-PMF and 130 (52%) post-PV/ET MPN-BP; among the latter, 60 patients had post-PV (32 without documented MF phase) and 70 post-ET (39 without documented MF phase) MPN-BP. Among all evaluable cases, at the time of leukemic transformation, 34% displayed red cell transfusion dependency, 58% platelet count <100 x 10(9)/L, 37% leukocyte count >25 x 10(9)/L, 71% PB blasts ≥20%, 87% BM blasts ≥20%, 81% abnormal karyotype, 57% unfavorable karyotype and 68% JAK2 V617F mutation. Compared to post-PMF, post-PV/ET MPN-BP cases displayed female preponderance (p=0.04), lower transfusion need (p=0.0004), higher platelet count (p=0.004) and lower circulating blast count (p=0.005), but similar karyotype profile (p=0.3).
Survival trends over time: After a median follow-up of 3 months (range 0-122), 226 (91%) deaths were recorded. Median overall survival (OS) was 3.6 months with 1- and 3-year survival rates of 17% and 6%, respectively; survival trends appeared significantly better (p=0.002) for patients diagnosed in the year 2000 and beyond, but with no additional improvement in more recent years (1-year survival rates for diagnosis before 2000 vs 2001-2009 vs 2010 and beyond were 5% vs 17% vs 20%, respectively).
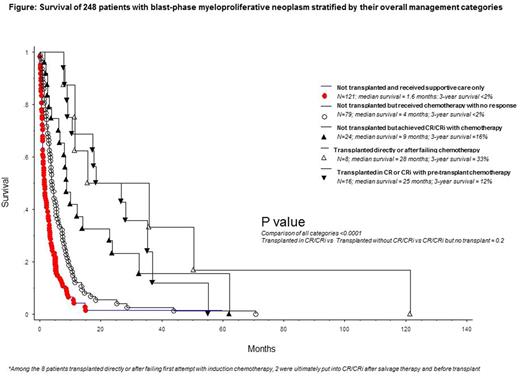
Treatment outcome and predictors of survival: Treatment response was assessable in 122 patients including 66 treated with acute myeloid leukemia (AML)-like induction chemotherapy, 26 with hypomethylating agents (HMA) and 30 with other agents; the respective complete remission (CR) rates were 35%, 4% and 3%; an additional 24% of patients who received AML-like induction chemotherapy achieved CR with incomplete blood count recovery (CRi) and their survival was similar to those with CR (p=NS); CRi was not seen with HMA. ASCT was reported in 24 patients and included 17 patients who received AML-like induction chemotherapy and 18 patients who were in CR or CRi, at time of transplant. In multivariable analysis, platelets <100 x 10(9)/L (HR 1.7; 95% CI 1.0-2.7) and unfavorable karyotype (HR 2.3; 95% CI 1.3-4.3) predicted inferior and achievement of CR/CRi (HR 0.4; 95% CI 0.2-0.7) superior survival; additional significance was not evident for ASCT (p=0.09). A composite assessment revealed that short-term survival was similarly and positively affected by either achieving CR/CRi or receiving ASCT, but the benefit did not endure (Figure).
AGIMM cohort (n=171):
An external cohort of 171 patients from AGIMM was used to validate most of the aforementioned observations from the Mayo Cohort (details to follow at time of presentation).
Conclusions: CR or CRi is achievable in MPN-BP, but only with AML-like induction chemotherapy and not with HMA; achievement of CR/CRi was associated with improved short-term survival, regardless of consolidation with ASCT. Outcome after ASCT was not affected by pre-transplant chemotherapy and neither ASCT nor achievement of CR/CRi resulted in durable survival benefit.
Passamonti: Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Speakers Bureau. Rambaldi: Novartis, Roche/Genentech, Amgen, Italfarmaco: Consultancy; Novartis, Amgen, Celgene, Sanofi: Other: Travel, Accomodations, Expenses. Vannucchi: Novartis: Honoraria, Speakers Bureau; Shire: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal